• In Business Since 2008
4199 Campus Drive,
Suite #550 Irvine CA, 92612
TMS Breakthrough for Major Depressive Disorder
Major Depressive Disorder (MDD) is a mental health condition that affects millions of people worldwide. It is characterized by persistent feelings of sadness, hopelessness, and worthlessness, among other symptoms. While there are many treatment options available for MDD, some patients do not respond well to traditional treatments such as medication and therapy.
How does TMS treat Major Depressive Disorder
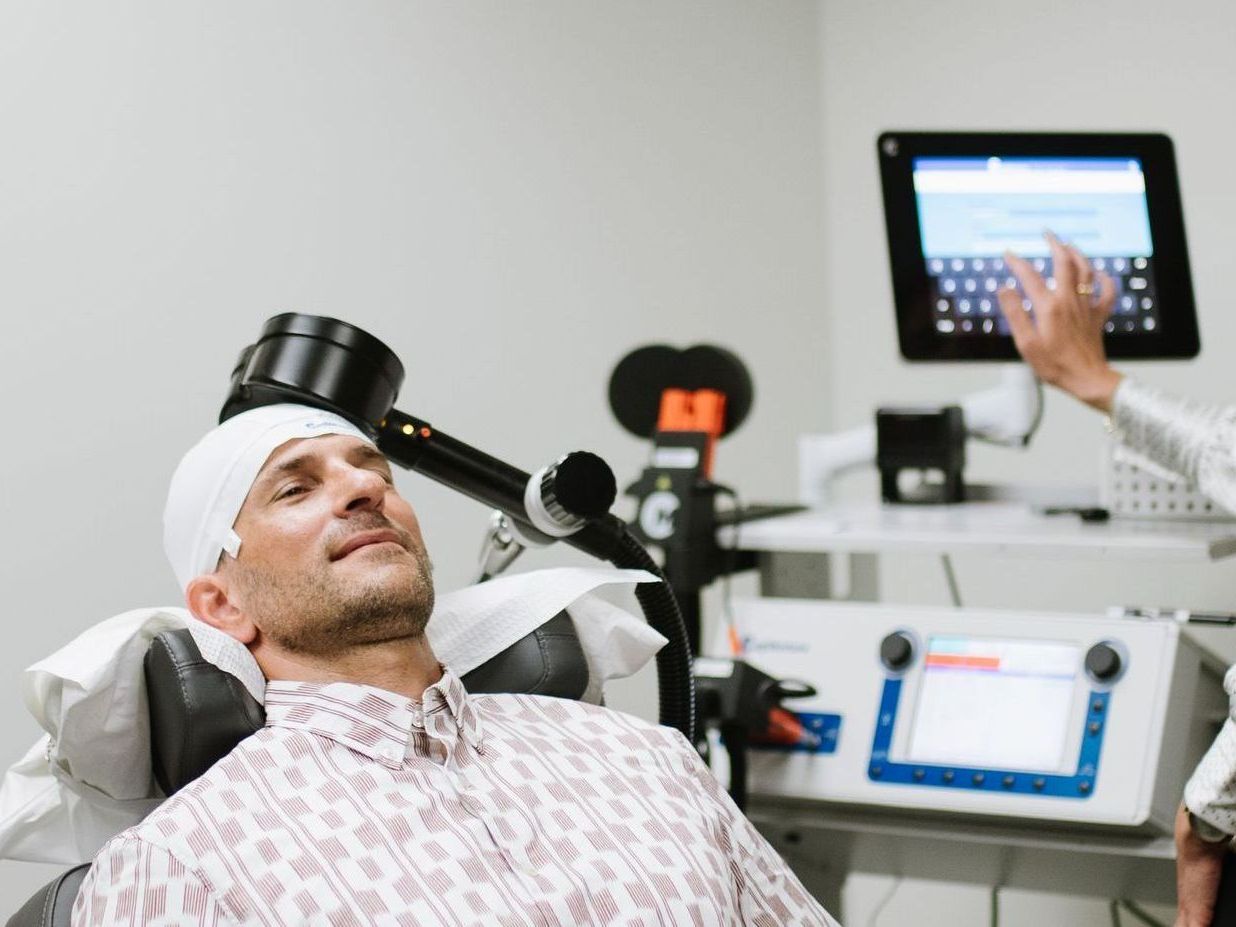
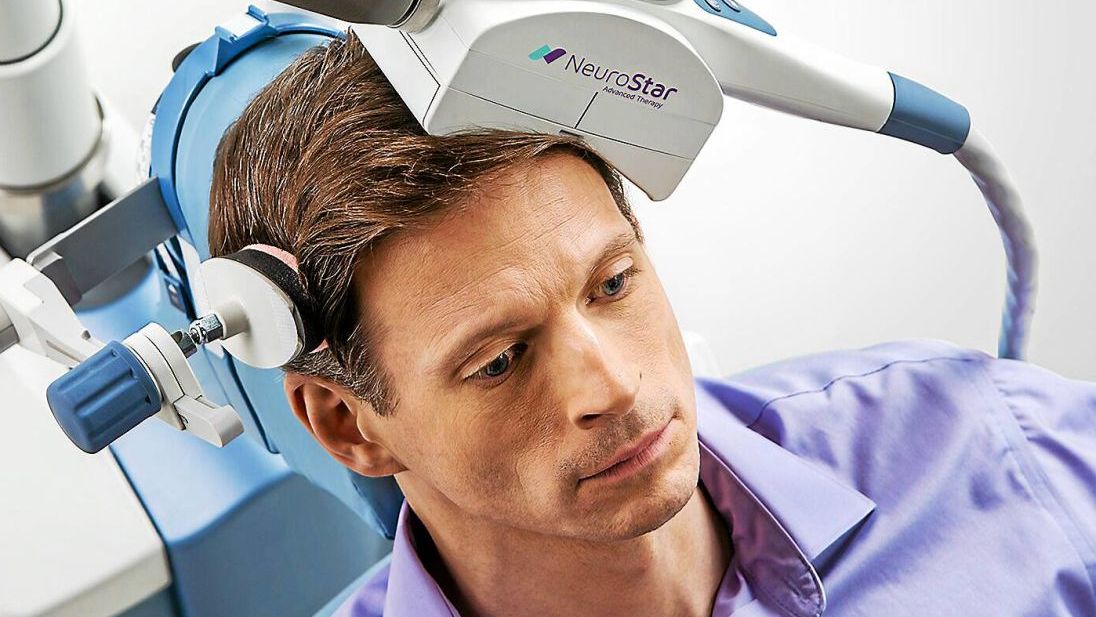
Transcranial Magnetic Stimulation (TMS) has emerged as an effective treatment option for MDD. TMS is a non-invasive procedure that uses magnetic fields to stimulate nerve cells in the brain. It has been approved by the FDA for the treatment of MDD for several years, but the number of illnesses treated and the treatment protocols continue to evolve.
SAINT™ TMS Breakthrough System
Recently, the FDA has approved the SAINT™ Neuromodulation System for the treatment of MDD in adults who have failed to achieve satisfactory improvement from prior antidepressant medications in the current episode. This breakthrough device designation and clearance represent a significant development for TMS treatment.
How Long Does SAINT™ take to see results
The current TMS treatment approach for adults with depression can take up to six weeks of treatments before depression will respond. However, the new potential approach can reduce depression response within five days. This is an exciting trend for the effectiveness of TMS treatment for depression.
The SAINT™ Neuromodulation System is designed to deliver TMS treatment in a more targeted and precise manner. This new treatment protocol uses a series of magnetic pulses to stimulate specific regions of the brain associated with depression. By targeting these regions more precisely, the SAINT™ Neuromodulation System can achieve faster and more effective results for patients.
While the SAINT™ Neuromodulation System is a significant development for TMS treatment, it does require additional equipment to be added to the TMS machine located at treatment centers. Clinics and doctors will need to invest in this new equipment to provide the SAINT™ Neuromodulation System treatment to their patients.
How we aim to our improve TMS service
Dr. Roula Creighton, a TMS expert at Irvine Center Location, is closely tracking this development and when the use of this new TMS treatment protocol will be available to the general public. She will be keeping her patients updated on any new developments in TMS treatment and what it means for their treatment options.
In conclusion, the FDA's approval of the SAINT™ Neuromodulation System is an exciting development for TMS treatment for MDD. This new treatment protocol can achieve faster and more effective results for patients who have failed to respond to traditional treatments. While the new protocol requires additional equipment to be added to TMS machines, it is a significant step forward in the treatment of MDD. Patients with depression who are considering TMS treatment should consult with a qualified physician to determine if this new protocol is an option for them.
RECENT POST:
Get latest updates Subscribe to Our Newsletter
Newsletter Subscription
We will get back to you as soon as possible.
Please try again later.
About Us
Irvine Psychiatry and TMS is Orange County's leading provider of cutting-edge & expert psychiatric care. We offer a variety of treatment options to meet each individual's needs.
Useful Links
Services
Irvine Psychiatry and TMS Dr. Roula Creighton
Phone: (949) 202-7566
Fax: (949) 437-3428
Email: admin@irvinepsychiatry.com
Location: 4199 Campus Drive, Suite #550 Irvine, California 92612
Irvine Psychiatry and TMS | Powered by Vulcan